M. Holtzmann, K. Leben, A. N. Macario Perera Laxgang
Privatärztliche Ambulanz für Venenheilkunde, Stuttgart
Visualization and Objectivization of Subfascial Edema with Sonography: The
Sonographic Muscle Compartment Scan.
M. Holtzmann, K. Leben, A. N. Macario Perera Laxgang
Privatärztliche Ambulanz für Venenheilkunde, Stuttgart
Visualization and Objectivization of Subfascial Edema with Sonography: The
Sonographic Muscle Compartment Scan.
Abstract
Little is known about subfascial edema. For 50 to 100 years palpatory detection has belonged to the standard phlebologic repertoire. Developing imaging diagnostics has almost completely displaced these manual skills. Finally, the lack of objectivizability has allowed subfascial edema to disappear from science and textbooks.
At the beginning of 2019 we discovered and developed a simple, inexpensive, reproducible, and documentable sonographic methodology to visualize subfascial edema unambiguously and thereby to objectivize all phlebologic pathologies, probably for the first time. For that reason the fascia compartment scan is our first-line screening and therapy-monitoring instrument (8). Moreover, as a final consequence is the first concept of a definitive preventive checkup for thrombosis. In this work we present the developed diagnostic methodology of the sonographic muscle compartment scan and a semiquantitative score for grading subfascial edema.
Keywords
Subfascial edema, sonographic muscle compartment scan, echo structure alterations, elevation of echogenicity, venous hypertension, proteoglycans, swelling in the posterior deep compartment
Background
All venous diseases rest on a disturbance of venous backflow (6). Impairments of venous recirculation trigger congestive conditions that in turn induce pre- and subfascial edema (14).
Of these two types of edema, subfascial edema plays the more serious role because it affects the more significant deep venous system. Detection of this endofascial swelling condition is of the highest interest from a therapeutic point of view (14).
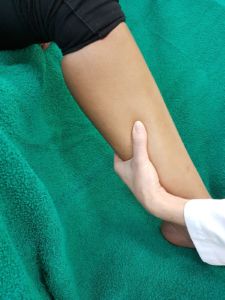
For the want of other possibilities for diagnosis of subfascial edema, our predecessors in venous medicine relied on their sense of touch. The deep venous system itself is, however, not accessible to the palpating hand, except the fascia surrounding the neurovascular bundles, stretched by the improved exfiltration (as it was thought) caused by the elevation of venous pressure due to congestion. By the so-called “subtle palpation according to Fischer and Haid,” the palpating hand feels the tension field of the swollen deep flexor compartments, detectable by touch (6). The patient confirms the palpatory finding by stating a concurring tenderness to pressure.
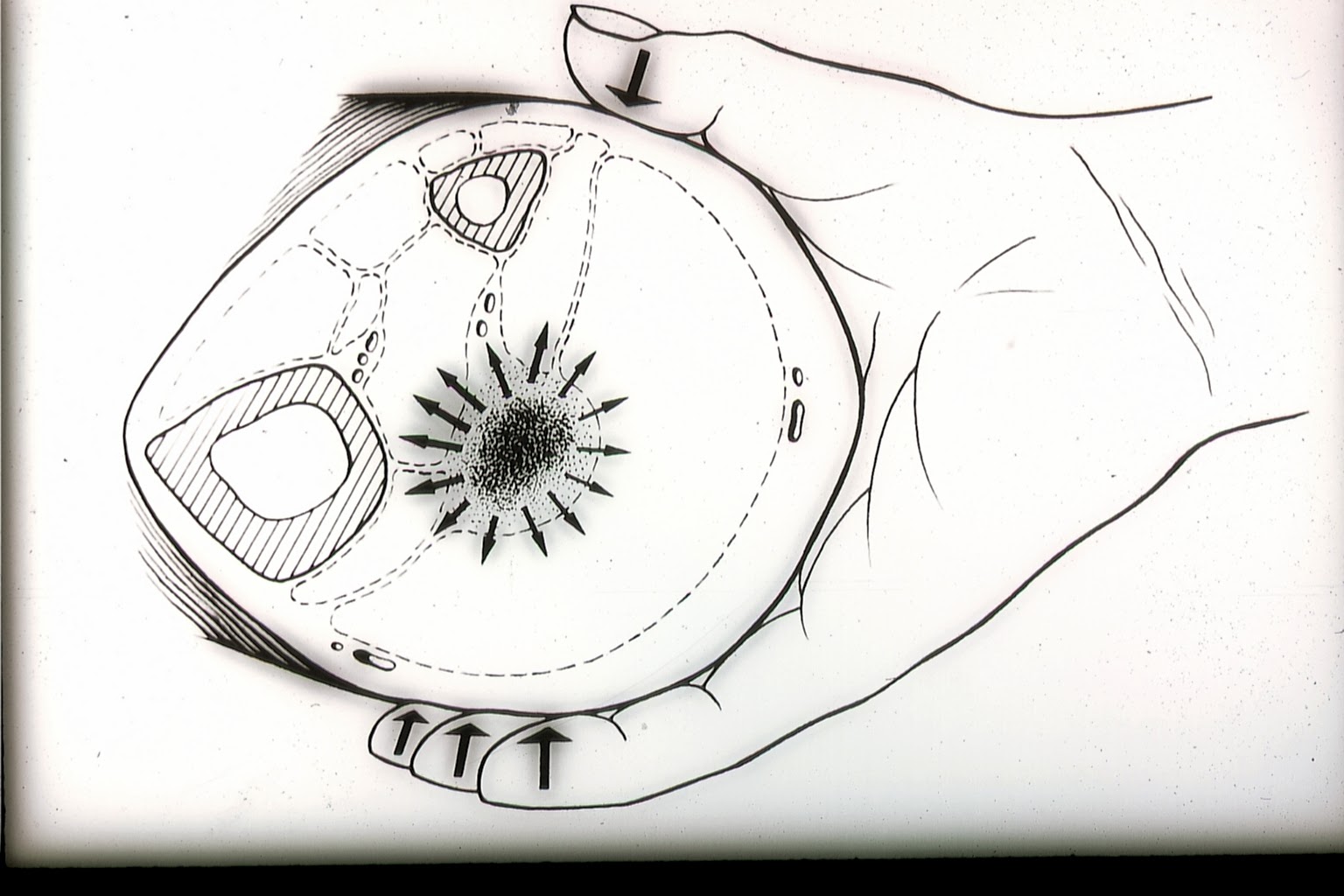
Fig. 1: The “subtle palpation according to Fischer and Haid” is a clinical investigative method for detection of subfascial edema. In the positive case, elevated turgor in the posterior deep compartment is felt as a characteristic hardening, painful to pressure.
(Haid-Fischer F., Haid H. Venenerkrankungen. Phlebologie für Klinik und Praxis. Georg Thieme Verlag, Stuttgart, New York. 1985.)
Fig. 2: If the palpating hand encounters a subfascial edema (the theoretic preliminary stage of venous thrombosis), the chance of an early preventive therapy opens up.
One must free oneself of the idea that with phlebitis only a local attack on the vein is involved and consider that the surrounding tissue must also be accordingly diseased and the circulatory impairment affects the entire extremity (6).
The different palpatory qualities are an expression of the tissue altered by chronic venous congestion or by other circulatory regulation impairments. Palpation of the perivascular space has as its goal to detect the soft tissue structural alterations of the venous wall as well as its environment, proceeding from or subsequent to an acute venous thrombosis.
However, little is known about the palpable substrate. The early pathologists Olow (1933) (11), Rössle (1937) (17), Voegt (1937) (19), Neumann (1937, 1938) (10), and Brass (1941, 1949) (2), using microscopic and histopathologic methods, sought soft tissue structure alterations that proceed from or follow a thrombosis. They described nonspecific vasal and perivasal tissue reactions due to congestion as well as alterations in the associated muscle parts due to circulation. In particular Rössle (1937) found alterations of the neighboring muscle fibers surprisingly frequent with venous thrombosis in the calves. He was sure that venous congestions lead to muscular alterations (16). His student Voegt found similar microscopic alterations in the calf muscles in patients with longer bed rest (18).
The dilemma of manual diagnosis is the necessary long experience of the investigator, coupled with arguably a certain investment and love for sensitive touch (6). From that it is understandable that palpatory findings were not altogether recognized in the absence of the possibility of objectivization.
The trained and experienced diagnostician was, however, in a position to “see” with fingertips in the depths of the tissue and to differentiate the finest distinctions in tissue. In this publication we can show that the compartment turgor elevation, palpable with tenderness, induced by venous congestion in the posterior deep compartment of the lower leg, the subfascial edema, is visible (myo)sonographically as a characteristic echo structure alteration (Tbl 1). We have named the investigation method published here the sonographic muscle compartment scan (8). With it, subfascial edema can for the first time be visualized, objectivized, and graded.
Materials and Methods
Ultrasound instruments used: GE Logiq S8, GE Logiq S7 Expert, GE Logiq S7 Pro, Samsung HS 60
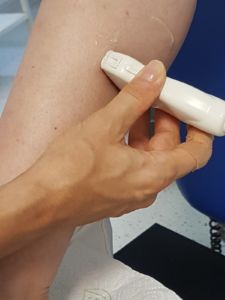
Recommended instrument settings: A standardized, constant setting of the sonography instrument as well as a standardized guidance of the ultrasound transducer are an unconditional requirement in the muscle compartment scan to obtain valid results.
The investigation should be undertaken with a linear transducer (15). This offers the advantage of an image true to the geometry.
- The multifrequency linear transducer with GE instruments (9L) is set to a center frequency of 8.5 MHz.
- The LA2-9A linear transducer of the Samsung HS 60 is set to a center frequency of 9 MHz “resolution.”
- A high dynamic range should be chosen (96 dB with GE, 120 Dynamic Range with Samsung) to make many gray levels possible.
- Reduce transmission power; recommended to 60%.
- Set the depth-dependent amplification (time gain compensation, TGC) equally.
- False color is advisable to make it easier to distinguish gray levels.
- Strictly perpendicular setup of the transducer. Insonation that is not exactly perpendicular alters the presentation of the muscle structure. Weak angular fluctuations already entail strong alterations in echogenicity. The technique with myosonography is analogous (1, 3, 5, 7).
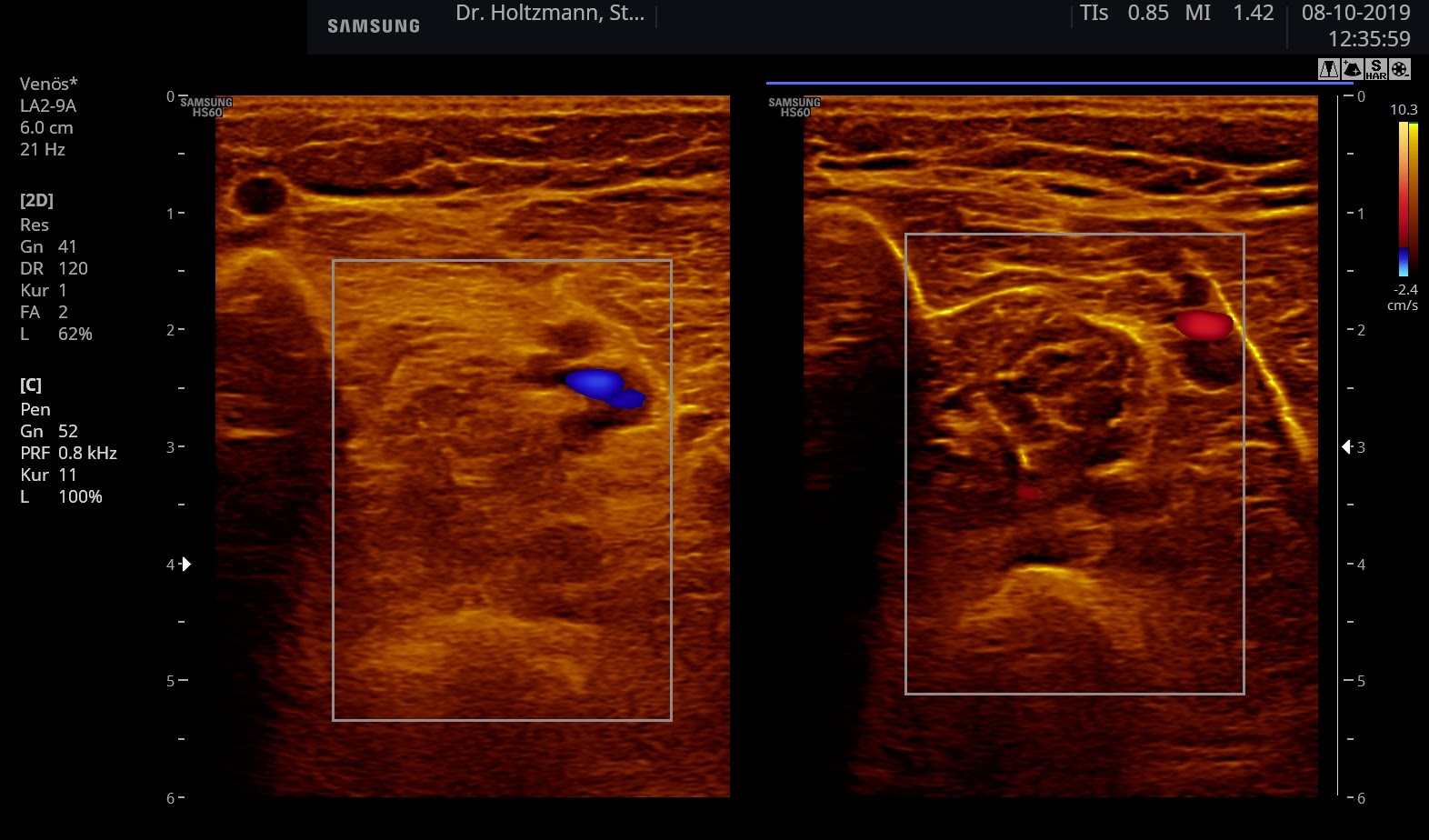
- Color Doppler mode turned on. With the size and position of the color window the region of interest (ROI) is marked.
- Split screen mode (right/left mode, dual mode) for direct side-by-side comparison. Both lower leg transverse sections are so juxtaposed, by which the unaffected or less affected extremity serves as a reference.
Transducer guidance and positioning:
- Sitting patient. The patient’s musculature is relaxed. Tensing of the muscle elevates the muscle diameter and reduces the echogenicity.
- The nose of the transducer points ventrally.
- The standard section level is the transverse section through the deep flexor compartments at about the transition of the medial gastrocnemius into the Achilles tendon.
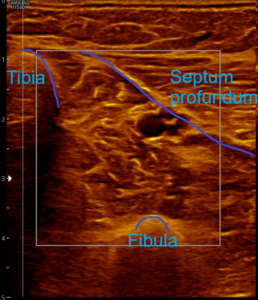
- Position the transducer so that the image boundary occurs on the left through the tibia, on the right through the lamina profunda (septum profundum), or the upper flexor compartment, and the deep boundary through the fibula.
- Exact orthograde insonation angle! Place the transducer without pressure (to avoid artefacts) (1, 3, 5, 7).
- The investigation should always take place bilaterally as a systematic comparison of sides, as the individual aspect is crucial. From myosonography we know that the muscular echo intensities vary considerably between individuals and from muscle to muscle within an individual. Even within a single muscle the echo intensities can be different in the different bellies.
Results
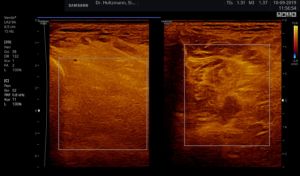
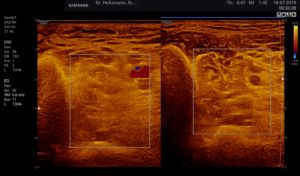
Without palpatory findings, healthy musculature in the posterior deep compartment appears almost echo-free or with low to medium echogenicity in the right/left mode (see Fig. 5). Fibroadipose septa and perimysia are visibly echo-rich. The muscles are surrounded by reflection-rich epimysia, which, however, are seldom delimitable in the posterior deep compartment, as part of the reflected sound waves miss the transducer because of the insonation angle and thereby are no more available for image generation. In transverse muscle section the starry sky phenomenon (reflection-rich, shining epimysia, perimysia, and vascular connective tissue within the “dark” echo structure of the musculature) appear (3, 5, 12, 15, 20, 21).
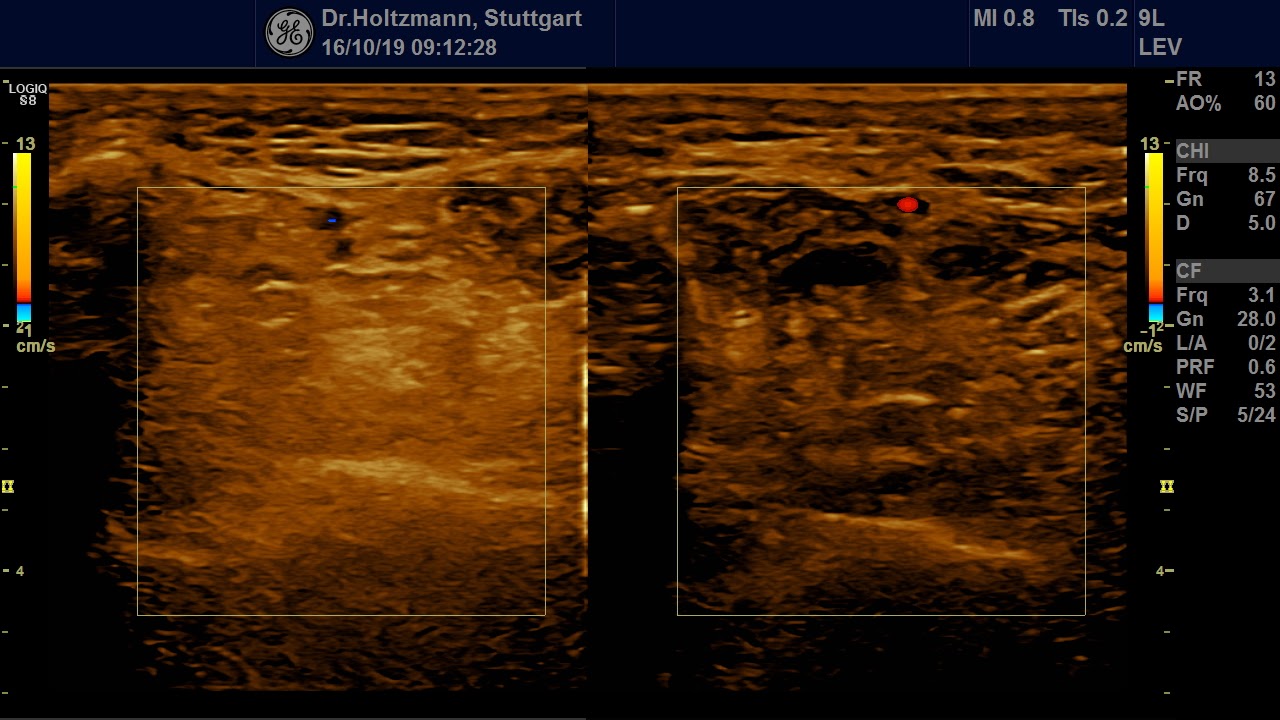
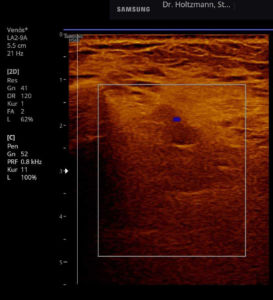
Fig. 5: Left and right transverse section through the deep flexor compartment presented side by side in split screen. On both sides, normal echo structure with negative palpatory finding. Exclusion of a subfascial edema.
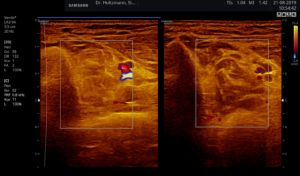
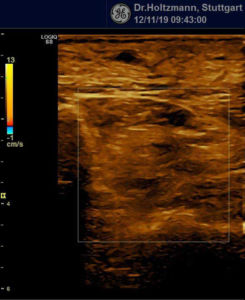
If a tender swelling, the subfascial edema, is detected in the posterior deep compartment of the lower leg by subtle palpation according to Fischer and Haid (6), the following demonstrated characteristic sonographic echo structure alterations can regularly be recognized. Thereby the preservation of the above described constant basic sonographic settings is crucial. The sonographic echo structure alterations correlate in their expression with the intensity of the palpatory findings and so can be graded.
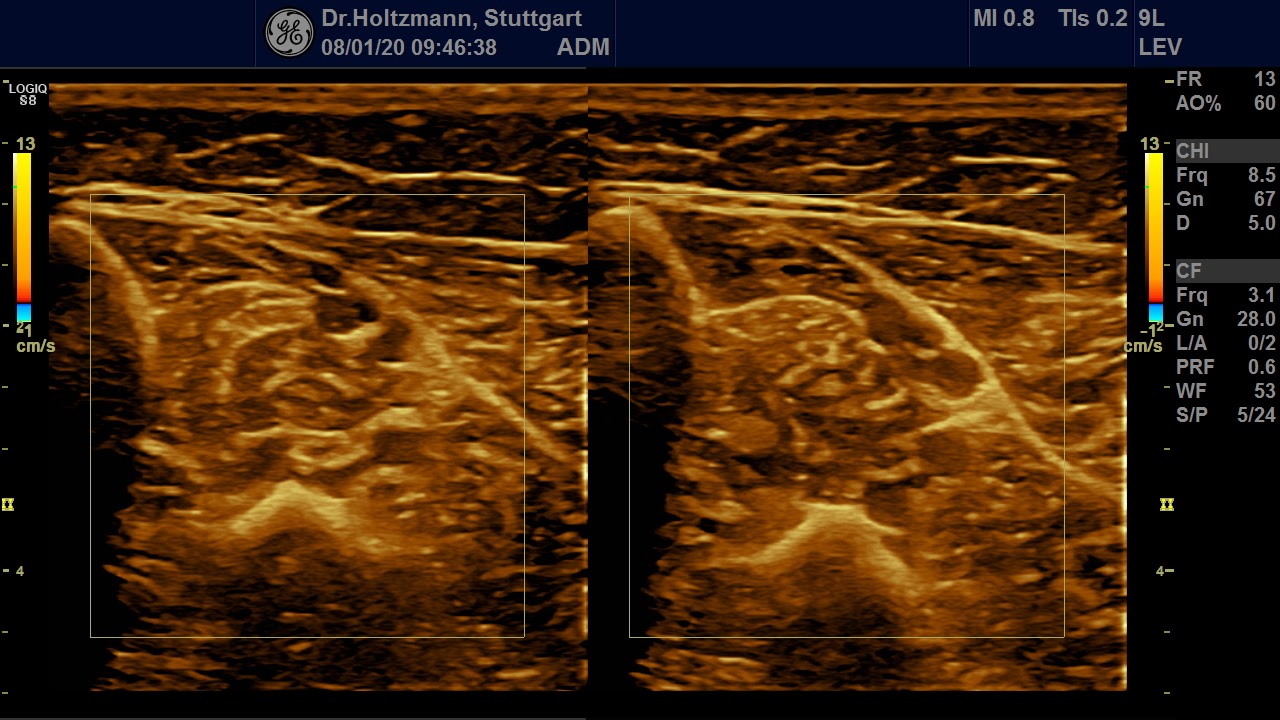
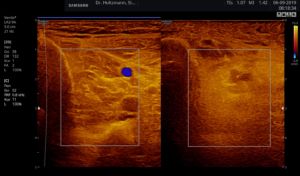
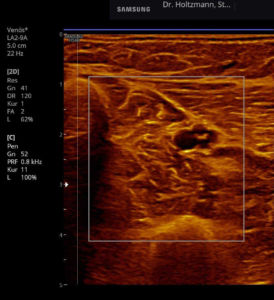
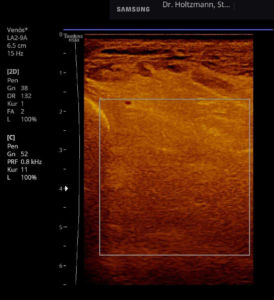
Fig. 6: Split screen setting with muscle compartment scan with positive palpatory findings in the left lower leg. On the right, without palpatory finding, the echo texture is normal. In the left transverse section, washed-out, not sharply delimited, homogeneous zones of middle density are recognized.
Elevated impedance differences, or increased jumps in impedance, lead like a grainy, rough boundary surface to an artefact of similar scattering. The impression of a washed-out echo texture arises.
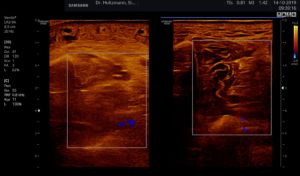
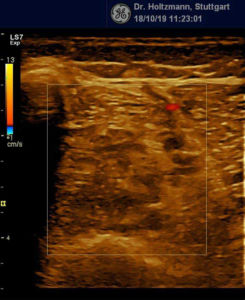
Fig. 7: Left: Subfascial edema with positive palpatory finding. Sonographically washed-out, confluent zones with raised echogenicity.
Right: Normal echo texture.
The pathologic echogenicity alterations with neurogenic and myogenic injuries are to be observed in differential diagnosis (3, 5, 12, 15, 20, 21). They can suggest a subfascial edema with the identical echo structure alterations.
To evaluate the expression of subfascial edema in a standardized way, we have developed a score. It facilitates therapy monitoring (with nonyielding compression media) as well as diagnostic screening, even with a view to a definitive preventive checkup for thrombosis.
Tbl 1: Semiquantitative Score for Sonographic Grading of Subfascial Edema
Peculiarity of subfascial edema grade 0
Normal, poorly reflecting, low echogenicity of the compartment musculature (flexor digitorum longus, tibialis posterior, flexor hallucis longus). Reflection-rich epimysia, perimysia (septa), and vascular connective tissue in “dark” echostructure of the musculature = “starry sky phenomenon.”
Peculiarity of subfascial edema grade 0
Normal, poorly reflecting, low echogenicity of the compartment musculature (flexor digitorum longus, tibialis posterior, flexor hallucis longus). Reflection-rich epimysia, perimysia (septa), and vascular connective tissue in “dark” echostructure of the musculature = “starry sky phenomenon.”
Discussion
Our sonographic diagnostics vitiates the current working hypothesis that through chronic venous congestion conditions, following a pressure gradient, a filtration of intravasal fluid into the interstitial space is the cause of subfascial edema (13).
Classic edema is defined as the exit of fluid from the vascular system and its accumulation in the extravasal interstitial space. These edemas make an impression in ultrasound through free fluid in the intercellular gaps, by which the B-image is streakily echo-free and thereby darker. As example, recognizable in prefascial or suprafascial edema.
Subfascial edema is consequently no classic edematous swelling, as sonographically no free fluid is detectable in the extravasal space. In our investigations no reduction, but rather a characteristic increase, of echogenicity appears, with brightening of the B- image.
The swelling that our phlebologic predecessors palpated and named subfascial edema must thus be of another genesis. Consequently the early pathologists cited above (2, 10, 11, 17,19) were not so wrong in their search for histopathologic alterations that would objectivize subfascial edema (4). They described no edema in the sense of an extravasal accumulation of fluid but rather unspecific muscular structure alterations that could correspond to our sonographic echogenicity alterations.
The echo structure alterations we have described in the case of a palpable and tender swelling of the deep flexor compartment can be traced back with the greatest likelihood to a biochemical alteration of the extracellular matrix as a reaction to venous hypertension. Thereby the proteoglycans in the amorphous ground substance of the loose connective tissue come to the fore. It is known that they can react with an increase in volume (swelling) and consequently with an increased echogenicity.
Loose connective tissue consists histologically of motile and resident cells that become embedded in an extracellular matrix. The extracellular matrix together with the cells forms a three-dimensional meshwork whose interstitial spaces is filled with the aforementioned proteoglycans.
Proteoglycans are very large molecules that consist of ~ 95% carbohydrates and ~ 5% proteins. The chemical properties correspond on the basis of the high carbohydrate content to those of polysaccharides. The prosthetic groups of proteoglycans are repeating disaccharide units. These are mostly sulfated, which causes the strongly negative charge and thereby the high water-binding capacity. With their high water-binding capacity they fulfill, among others, a space-filling function as the ground substance of the extracellular matrix in cartilage, tendons, and additionally as lubricant in joints (chondroitin sulfate, keratan sulfate). Thereby the strongly swelling proteoglycans serve to take up and distribute compressive forces.
The congestively swelling proteoglycans in the extracellular matrix gradually alter the echo structure of the deep flexor musculature and, in the swollen condition, strengthen the sound reflection. Elevated impedance differences, or increased jumps in impedance, lead to a scattering as on a grainy, rough boundary surface. The echo texture acts to wash out (9) and can grow according to the intensity of the subfascial edema to a complete loss of the normal echo texture.
The sonographic image of subfascial edema shows the same increase in echogenicity as the myosonographic image of muscular edema (14) and the same sonographic characteristics (diffuse elevated echogenicity) as with acute and chronic compartment syndrome (3, 5, 12, 15).
One can generalize that with increasing intracompartmental pressure the muscular echo intensity rises (15).
The pathologic echogenicity alterations with neurogenic and myogenic injuries are to be observed in differential diagnosis (3, 5, 12, 15, 20, 21). They can suggest a subfascial edema with the identical echo structure alterations.
Only for very experienced palpatory diagnosticians has it previously been possible to distinguish a neurogenic complaint complex (emanating from the lumbar spinal cord) with protective muscular tension from the picture of venous complaint with subfascial swelling. Along with similar pain symptoms, both present a positive palpatory finding. With the sonographic muscle compartment scan, an unambiguous differentiation is easily possible for the first time. Neurogenic causes (lumbar spine syndrome with irritation of the tibial nerve) show in the acute stage almost no echo structure alteration; venous causes (subfascial edema) show echogenicity alterations of grade 1 through 4. All quests of a way to objectivize subfascial edema that were carried out according to the cited early pathologists have failed; the quests in the radiologic field (Haid-Fischer, 1970) as well.
In 2017 Stumptner (18) published a work in which subfascial edema would be objectivized using magnetic resonance tomography (MRT). In his presentation technique all tissue segments of the lower leg transverse section are affected by MRT signal alterations. He proceeds thence that venous hypertension of the deep veins leads to a subfascial increase in water retention in the patients’ lower leg. The original palpable subfascial edema extends, however, only over the posterior deep compartment (6). In our sonographic diagnostic method, the characteristic echogenicity alterations are restricted specifically to these central neurovascular bundles; to the place where a tender swelling can also be detected with the subtle palpation technique. On this basis we are skeptical whether the MRT signal alterations shown by Stumptner illustrate the phlebologic phenomenon that is named subfascial edema.
Conclusion
The sonographic muscle compartment scan has proved itself in our outpatient practice since 2019 as a reliable, reproducible, documentable instrument for diagnosis and monitoring of subfascial edema. This investigation method does not impose high demands on either the investigator or on the instrument technician.
As we consider subfascial edema as the central substrate of venous pathology, the sonographic muscle compartment scan has become our phlebologic first-line screening and therapy-monitoring instrument (with inelastic compression bandages (8)). As a final consequence it also represents an objective preventive checkup, with a view to the origin of venous thrombosis.
Literature
- Ackermann R. Sonographie traumatischer Veränderungen der Muskulatur. Braun B, Günther R, Schwerk WB (Hrsg.), Ultraschalldiagnostik. Lehrbuch und Atlas. Ecomed, Landsberg/Lech 1995;1-41.
- Brass K. Aufbau und Entstehung der Beinvenenthrombose. Frankfurt. Z. Path. 1941;56:74.
- Cartwright MS, Demar S, Griffin LP et al. Validity and reliability of nerve and muscle ultrasound. Muscle Nerve 2013;47:515–521.
- Fischer H. Eine neue Therapie der Phlebitis. Medizin Klinik 1910;30.
- Grimm A, Axer H, Quasthoff S. Methodik Muskelsonografie. In: Bischoff C, Buchner H, Hrsg. SOPs Neurophysiologische Diagnostik. Georg Thieme Verlag Stuttgart, 2018. doi:10.1055/b-005-149022.
- Haid-Fischer F, Haid H. Venenerkrankungen. Phlebologie für Klinik und Praxis. Georg Thieme Verlag, Stuttgart 1985.
- Harland U. Die Abhängigkeit der Echogenität vom Anschallwinkel an Muskulatur und Sehnengewebe. Z Orthop, 1988;126:117-124
- Holtzmann M, Leben K. Spezifischer biphasischer Druckaufbau unter nicht nachgiebigen Kompressionsverbänden beim intensiven Gehen. Vasomed 2020;2:54-60.
- Lutz HTH. Ultraschallfibel Innere Medizin. Springer Verlag Berlin, Heidelberg, 2007;122
- Neumann R. Ursprungszentren und Entwicklungsformen der Beinvenenthrombose. Virchows Arch. Path. Anat. 1938;301:708
- Olow R. zit. nach Rössle 1937. In Haid-Fischer F, Haid H. Venenerkrankungen. Phlebologie für Klinik und Praxis. Georg Thieme Verlag, Stuttgart 1985;59-60.
- Pillen S, Arts IM, Zwarts MJ. Muscle ultrasound in neuromuscular disorders. Muscle Nerve 2008;37:679–693
- Pschyrembel – Klinisches Wörterbuch. De Gruyter, Berlin 1998;1143
- Rabe E, Gerlach HE. Praktische Phlebologie. Georg Thieme Verlag, Stuttgart 2000;86
- Reimers CD, Gaulrapp H, Kele H (Hrsg.). Sonographie der Muskeln, Sehnen und Nerven. Untersuchungstechnik und Befundinterpretation. Deutscher Ärzte-Verlag, Köln 2004.
- Roberts CS et al. Diagnostic ultrasonography: applications in orthopedic surgery. Clin Orthop. 2002;401:248-264
- Rössle R. Über die Bedeutung und Entstehung der Waden-Venenthrombosen. Virchows Arch. path. Anat. 1937;300:180
- Stumptner T. Das subfasziale venöse Ödem im NMR. Phlebologie 2018;47: 205-209
- Voegt H. Veränderungen der Wadenmuskulatur bei Venenthrombose und langem Krankenlager. Virchows Arch. path. Anat. 1937;300:190
- Walker FO, Cartwright MS. Neuromuscular Ultrasound. Philadelphia: Elsevier Saunders; 2011
- Zaidman CM, van Alfen N. Ultrasound in the Assessment of Myopathic Disorders. J Clin Neurophysiol 2016;33:103-111.